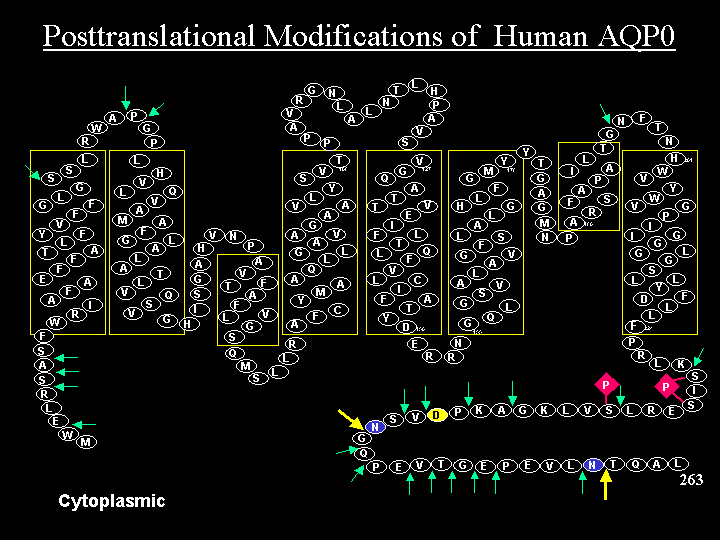
The Schey lab discovered lenticular Aquaporin-5 (AQP5) protein and also the majority of the posttranslational modifications (PTMs) present on the most abundant lens membrane protein, Aquaporin-0 (a.k.a. lens major intrinsic protein, MIP, or MP26). The major focus of our AQP research is to elucidate the role of these water channels in the lens microcirculation system, known to be essential for lens transparency. More specific goals include: determining the spatial localization of specific AQP modifications and how they alter AQP function, defining the mechanism of lens AQP5 trafficking, and identifying AQP-protein and AQP-lipid interactions. We also have an interest in global lens membrane proteome changes with age and in other membrane proteins such as Lim2 and connexins.
Techniques such as immunohistochemistry/high resolution confocal microscopy, spatially-resolved proteomics, crosslinking mass spectrometry (XL-MS), imaging mass spectrometry (IMS), native MS, top down MS, data independent acquisition (DIA) proteomics and hydrogen-deuterium exchange mass spectrometry (HDX-MS) are being used to address the aforementioned goals and to shed light on AQP structures and functions in the lens.

Related References
O’Neale CVT, Tran MH, Schey KL. Aquaporin-0-Protein Interactions Elucidated by Crosslinking Mass Spectrometry. Biochem. Biophys. Res. Commun., in press.
Petrova RS, Francis N, Schey KL, Donaldson PJ. Verification of the Gene and Protein Expression of the Aquaglyceroporin AQP3 in the Mammalian Lens. Exp. Eye Res., 240:109828, 2024.
Petrova RS, Nair N, Bavana N, Chen Y, Schey KL, Donaldson PJ. Modulation of the Membrane Trafficking of AQP5 in the Lens in Response to Changes in Zonular Tension is Mediated by the Mechanosensitive Channel TRPV1. Int. J. Mol. Sci., 24:9080, 2023.
Donaldson PJ, Petrova R, Nair N, Chen Y, Schey KL. Regulation of Water Flow in the Ocular Lens: New Roles for Aquaporins. J. Physiol., Oct 16:10.1113/JP284102, 2023.
Tornroth-Horsefield S, Chivasso C, Strandberg H, D’Agostino C, O’Neale CVT, Schey KL, Delporte C. Insight into the mammalian aquaporin interactome. Int. J. Mol. Sci., 23:9615, 2022.
Gletten RB, Cantrell LS, Schey KL. Lens aquaporin-5 inserts into bovine fiver cell plasma membranes via unconventional protein secretion. Invest. Ophthalmol. Vis. Sci., 63:5, 2022.
Harvey S, O’Neale C, Schey KL, Wysocki VW. Insight into the posttranslational modifications of tetrameric AQP0 isolated from bovine eye lens using native MS and surface-induced dissociation. Anal. Chem., 94:1515-1519, 2022.
Schey KL, Gletten RB, O’Neale CVT, Wang Z, Petrova RS, Donaldson PJ. Lens aquaporins in health and disease: Location is everything! Front. Physiol., 13:882550, 2022.
Chivasso C, Nesverova V, Jarva M, Blanchard A, Rose KL, Oberg FK, Wang Z, Martin M, Lhotellerie F, Zindy E, Junqueira B, Leroy K, Vanhollebeke B, Delforge V, Bolaky N, Perret J, Soyfoo MS, Moscato S, Baldini C, Chaumont F, Mattii L, Schey KL, Tornroth-Horsefield S, Delporte C. Unraveling human AQP5-PIP molecular interaction and effect on AQP5 salivary glands localization in SS patients. Cells 10:2108, 2021.
Giblin FJ, Anderson DMG, Han J, Rose KL, Wang Z, Schey KL. Acceleration of age-induced proteolysis in the guinea pig lens nucleus by in vivo exposure to hyperbaric oxygen: a mass spectrometry analysis. Exp. Eye Res., 210:108697, 2021.
Wang Z, Cantrell LS, Schey KL. Spatially-Resolved Proteomic Analysis of the Lens Extracellular Diffusion Barrier. Invest. Ophthalmol. Vis. Sci., 62:25, 2021.
Wang Z, Ryan DJ, Schey KL. Localization of the Lens Intermediate Filament Switch by Imaging Mass Spectrometry. Exp. Eye Res., 198:108134, 2020.
Petrova R, Bavana N, Zhao R, Schey KL, Donaldson PJ. Changes to zonular tension alters the subcellular distribution of AQP5 in regions of influx and efflux of water in the rat lens. Invest. Ophthalmol. Vis. Sci., 61:36, 2020.
Wang Z and Schey KL. Proteomic Analysis of S-Palmitoylated Proteins in Ocular Lens Reveals Palmitoylation of AQP5 and MP20. Invest Ophthalmol Vis Sci., 59:5648-5658, 2018.
Petrova R, Webb K, Vaghefi E, Walker K, Schey KL, Donaldson PJ. Dynamic Functional Contribution of the Water Channel AQP5 to the Water Permeability of Peripheral Lens Fiber Cells. Am. J. Physiol. 314:C191-C201, 2018.
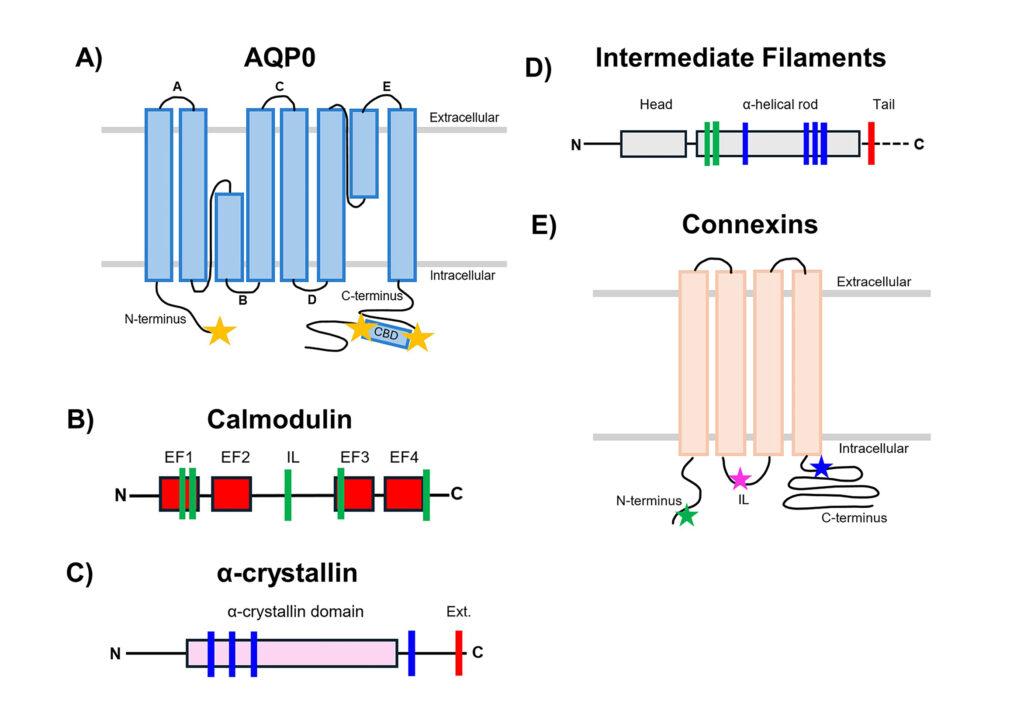
Wang Z and Schey KL. Identification of a Direct Aquaporin-0 Binding Site on Lens-Specific Cytoskeletal Protein Filensin. Exp. Eye Res. 159:23-29, 2017.
Schey KL, Petrova RS, Gletten RB, Donaldson PJ. The Role of Aquaporins in Ocular Lens Homeostasis. Int J Mol. Sci. 18:2693, 2017.
Gutierrez DB, Garland D, Schwacke JH, Hachey DL, Schey KL. Spatial Distributions of Phosphorylated Membrane Proteins Aquaporin 0 and MP20 Across Young and Aged Human Lenses. Exp. Eye. Res., 149:59-65, 2016.
Wenke JL, McDonald WH, Schey KL. Spatially-Directed Proteomics of the Human Lens Outer Cortex Reveals an Intermediate Filament Switch Associated with the Remodeling Zone. Invest. Ophthalmol. Vis. Sci., 57:4108-4114, 2016.
Wenke JL, Rose KL, Spraggins JM, Schey KL. MALDI Imaging Mass Spectrometry Spatially Maps Age-Related Deamidation and Truncation of Human Lens Aquaporin-0. Invest. Ophthalmol. Vis. Sci., 56:7398-7405, 2015.
Wang Z and Schey KL. Proteomic Analysis of Lipid Raft-like Detergent-Resistant Membranes of Lens Fiber Cells. Invest. Ophthalmol. Vis. Sci. 56:8349-8360, 2015.
Petrova RS, Schey KL, Donaldson, PJ, Grey AC. Spatial distributions of AQP5 and AQP0 in embryonic and postnatal mouse lens development. Exp. Eye Res., 132C:124-135, 2015.
Schey KL, Wang Z, Wenke J, Qi Y. Aquaporins in the Eye: Expression, Function, and Roles in Ocular Disease. Biochim. Biophys. Acta, 1840:1513-1523, 2014.
Schey KL, Grey AC, Nicklay JJ. Mass Spectrometry Analysis of Membrane Proteins: A Focus on Aquaporins, Biochemistry, 52:3807-3817, 2013.
Maddala R, Nagendran T, de Ridder GG, Schey KL, Rao PV. L-Type Calcium Channels Play a Critical Role in Maintaining Lens Transparency by Regulating Phosphorylation of Aquaporin-0 and Myosin Light Chain and Expression of Connexins, PLoS One, 8:e64676, 2013.
Wang Z, Han J, David LL, Schey KL. Proteomics and Phosphoproteomics Analysis of Human Lens Fiber Cell Membranes, Invest. Ophthalmol. Vis. Sci. 54:1135-1143, 2013.
Schey KL, Grey AC, Nicklay JJ. Mass Spectrometry Analysis of Membrane Proteins: A Focus on Aquaporins, Biochemistry, 52:3807-3817, 2013.
Grey, AC, Walker KL, Petrova RS, Han J, Wilmarth PA, David LL, Donaldson PJ, Schey KL. Verification and Spatial Localization of Aquaporin-5 in the Ocular Lens. Exp. Eye Res. 108:94-102, 2013.
Gutierrez DB, Garland D, Schey KL. Spatial analysis of human lens aquaporin-0 post-translational modifications by MALDI mass spectrometry tissue profiling, Exp. Eye Res., 93:912-920, 2011.
Truscott RJW, Comte-Walters S, Ablonczy A, Schwacke JH, Berry Y, Korlimbinis A, Friedrich MG, Schey KL. Tight binding of proteins to membranes from older human cells. AGE, 33:543-54, 2011.
Wang Z and Schey KL. Aquaporin-0 interacts with the FERM domain of ERM proteins in the ocular lens, Invest. Ophthalmol. Vis. Sci. 52:5079-5087, 2011.
Schey KL, Wang Z, Gutierrez DB, Wei J, Grey AC. Novel fatty acid acylation of lens integral membrane protein aquaporin-0. Biochemistry 49:9858-9865, 2010.
Wang Z, Obidike JE, Schey KL. Posttranslational Modifications of Bovine Lens Beaded Filament Proteins Filensin and CP49. Invest. Ophthalmol. Vis. Sci., 51:1565-74, 2010.
Wang Z and Schey KL. Phosphorylation and Truncation Sites of Bovine Lens Connexin 46 and Connexin 50, Exp. Eye Res., 89:898-904, 2009.
Grey AC, Li L, Jacobs MD, Schey KL, Donaldson PJ. Differentiation-dependent Modification and Sub-cellular Distribution of Aquaporin-0 Suggests Multiple Functional Roles in the Rat Lens, Differentiation, 77:70-83, 2009.
Korlimbinus A, Berry Y, Thibault D, Schey KL, Truscott RJW: Protein aging: Truncation of aquaporin 0 in human lens regions is an age dependent process that may contribute to barrier formation, Exp. Eye Res. 88:966-973, 2009.
Grey AC, Chaurand P, Caprioli, RM , Schey KL. MALDI Imaging Mass Spectrometry of Integral Membrane Proteins from Ocular Lens and Retinal Tissue, J. Proteom. Res., 8:3278-3283, 2009.
Lim J, Walker K, Sherwin T, Schey KL, Donaldson P. Confocal Microscopy Reveals a Zone of Membrane Remodelling in the Outer Cortex of the Human Lens, Invest. Ophthalmol. Vis. Sci., 50:4304-4310, 2009.
Lindsey Rose KM, Wang Z, Magrath GN, Hazard ES, Hildebrandt JD, and Schey KL. Aquaporin 0-Calmodulin Interaction and the Effect of AQP0 Phosphorylation, Biochemistry, 2008, 47:339-347.
Thibault DB, Gillam CJ, Han J, Schey KL. MALDI Tissue Profiling of Integral Membrane Proteins from Ocular Tissues, J. Amer. Soc. Mass Spectrom., 19:814-22, 2008.
Wang Z, Han J, Schey KL. Spatial Differences in an Integral Membrane Proteome Detected in Laser Capture Microdissected Samples, J. Proteome Res., 7:2696-702, 2008.
Rose KML, Gourdie RG, Prescott AR, Quinlan RA, Crouch RK and Schey KL. The C-terminus of lens aquaporin 0 interacts with the cytoskeletal proteins filensin and CP49, Invest Ophthalmol Vis Sci, 2006, 47:1562-1570.
Ervin LA, Ball LE, Crouch RK, Schey KL: Phosphorylation and Glycosylation of Bovine Lens MP20. Invest. Ophthalmol Vis Sci, 2005, 46:627-635.
Han J, and Schey KL. Proteolysis and mass spectrometric analysis of an integral membrane protein: Aquaporin 0. J Proteome Res., 2004, 3:807-812.
Ball LE, Garland DL, Crouch RK and Schey KL. Post-translational modifications of aquaporin 0 (AQP0) in the normal human lens: Spatial and temporal occurrence. Biochemistry 2004, 43:9856-9865.
Han J, Little M, David LL, Giblin FJ, and Schey KL. Sequence and peptide map of guinea pig aquaporin 0. Mol. Vis., 2004, 10:215-222.
Ball LE, Little M, Nowak MW, Garland DL, Crouch RK, Schey KL: C-terminally Truncated Aquaporin 0 (AQP01-243) Observed in the Aging Human Lens Remains Functionally Viable, Invest. Ophthalmol. Vis. Sci., 2003, 44:4820-4828.
Schey KL, Little ML, Fowler JG, Crouch RK: Characterization of Human Lens Major Intrinsic Protein Structure, Invest. Ophthalmol. Vis. Sci., 2000, 41:175-182.
Schey KL, Fowler JG, Shearer TR, David L: Modifications to Rat Lens Major Intrinsic Protein in Selenite-Induced Cataract, Invest. Ophthalmol. Vis. Sci., 1999, 40:657-667.
Swamy-Mhruthinti S, Schey KL: Mass Spectrometric Identification of In Vitro Glycated Sites of MIP. Curr. Eye Res., 1997, 16:936-941.
Schey KL, Fowler JG, Schwartz JC, Busman M, Dillon J, Crouch RK: Complete Map and Identification of the Phosphorylation Site of Bovine Lens MIP. Invest. Ophthalmol. Vis. Sci., 1997, 38:2508-2515.